Your 2cuso4 4ki 2cui i2 2k2so4 images are available in this site. 2cuso4 4ki 2cui i2 2k2so4 are a topic that is being searched for and liked by netizens today. You can Get the 2cuso4 4ki 2cui i2 2k2so4 files here. Get all free images.
If you’re looking for 2cuso4 4ki 2cui i2 2k2so4 images information connected with to the 2cuso4 4ki 2cui i2 2k2so4 keyword, you have come to the ideal blog. Our site frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
2cuso4 4ki 2cui I2 2k2so4. Oksidator reduktor hasil oksidasi dan hasil reduksi dapat ditentukan setelah seluruh atom tiap senyawa yang terlibat dalam reaksi diperiksa bilangan oksidasinya apakah bertambah atau menurun. با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. Ce2CuSO4 4KI 2Na2S2O3 - 2CuI 2K2SO4 Na2S4O6 2NaI I did another method of synthesising ceCuI by preparing it directly from its elements with ceNaI as the solvent for solid ceI2. 2 cu 2 s 6 o-2 4 4ki 2e- 2h 2 cu 1 i-1 2k 2 so 4 i 2 Balanced half-reactions are well tabulated in handbooks and on the web in a Tables of standard electrode potentials.
 In The Reaction 2cuso 4 4ki Rarr 2cu 2 I 2 I 2 2k 2 So From doubtnut.com
In The Reaction 2cuso 4 4ki Rarr 2cu 2 I 2 I 2 2k 2 So From doubtnut.com
2CuSo44kI–2CuII22K2So4 سئوال یك آزمایش طراحی نمایید كه به كمك آن بتوان درصد مس را در كات كبود تعیین كرد. 4KI 2CuSO4 2CuI I2 2K2SO4 2 ii Deduce the charge on the copper ion in CuI. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations. Ce2CuSO4 4KI 2Na2S2O3 - 2CuI 2K2SO4 Na2S4O6 2NaI I did another method of synthesising ceCuI by preparing it directly from its elements with ceNaI as the solvent for solid ceI2. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator.
1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol.
Unsur iodin dalam senyawa dapat ditemukan dengan biloks dari -1 sampai 7. Enter an equation of a. Click hereto get an answer to your question In the reaction 2CuSO4 4KI Cu2I2 2K2SO4 I2 the equivalent weight of CuSO4 is. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator. 4KI 2CuSO4 2CuI I2 2K2SO4 2 ii Deduce the charge on the copper ion in CuI. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol.

با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. 4KI 2CuSO4 2CuI I2 2K2SO4 2 ii Deduce the charge on the copper ion in CuI. 2CuSo44kI–2CuII22K2So4 سئوال یك آزمایش طراحی نمایید كه به كمك آن بتوان درصد مس را در كات كبود تعیین كرد. CuSO4 KI CuI I2 K2SO4 CuSO4 KI CuI K2SO4 KI3 CuSO4 KI CuI2 K2SO4 Instructions and examples below may help to solve this problem You can always ask for help in the forum Instructions on balancing chemical equations. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator.
 Source: yumpu.com
Source: yumpu.com
Unsur iodin dalam senyawa dapat ditemukan dengan biloks dari -1 sampai 7. Mw 1595g mol-1. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations. Tentukan apakah reaksi 2CuSO4 4KI 2CuI I2 2K2SO4. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator.

1 1 iii In terms of electron transfer explain why copper is reduced in this reaction. Tentukan apakah reaksi 2CuSO4 4KI 2CuI I2 2K2SO4. CuSO4 KI CuI I2 K2SO4 CuSO4 KI CuI K2SO4 KI3 CuSO4 KI CuI2 K2SO4 Instructions and examples below may help to solve this problem You can always ask for help in the forum Instructions on balancing chemical equations. 2CuSO4 4KI 2CuI I2 2K2SO4 Cu 2 1 reduksi I -1 0 oksidasi Reduktor KI Oksidator CuSO4 Hasil reduksi CuI Hasil Oksidasi I2 b. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol.
 Source: yumpu.com
Source: yumpu.com
Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations. با تشكر - تبیان. Oksidator reduktor hasil oksidasi dan hasil reduksi dapat ditentukan setelah seluruh atom tiap senyawa yang terlibat dalam reaksi diperiksa bilangan oksidasinya apakah bertambah atau menurun. با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. 2CuSO4 4KI 2CuI I2 2K2SO4 Experts please explain how Cu2 changed to Cu1 in above reaction - Science - Chemical Reactions and Equations.
 Source: doku.pub
Source: doku.pub
2 cu 2 s 6 o-2 4 4ki 2e- 2h 2 cu 1 i-1 2k 2 so 4 i 2 Balanced half-reactions are well tabulated in handbooks and on the web in a Tables of standard electrode potentials. Ce2CuSO4 4KI 2Na2S2O3 - 2CuI 2K2SO4 Na2S4O6 2NaI I did another method of synthesising ceCuI by preparing it directly from its elements with ceNaI as the solvent for solid ceI2. با تشكر - تبیان. These tables by convention contain the half-cell potentials for reduction. CuSO4 KI CuI I2 K2SO4 CuSO4 KI CuI K2SO4 KI3 CuSO4 KI CuI2 K2SO4 Instructions and examples below may help to solve this problem You can always ask for help in the forum Instructions on balancing chemical equations.

4KI 2CuSO4 —– 2CuI I2 2K2SO4. Click hereto get an answer to your question In the reaction 2CuSO4 4KI 2Cu2I2 2K2SO4 the equivalent weight of CuSO4 will be. Tentukan apakah reaksi 2cuso4 4ki 2cui i2 2k2so4 merupakan reaksi redoks atau bukan. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol. 2CuSO4 4KI 2CuI I2 2K2SO4 Experts please explain how Cu2 changed to Cu1 in above reaction - Science - Chemical Reactions and Equations.
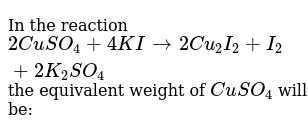 Source: doubtnut.com
Source: doubtnut.com
Tentukan apakah reaksi 2CuSO4 4KI 2CuI I2 2K2SO4. CuSO4 KI CuI I2 K2SO4 CuSO4 KI CuI K2SO4 KI3 CuSO4 KI CuI2 K2SO4 Instructions and examples below may help to solve this problem You can always ask for help in the forum Instructions on balancing chemical equations. Ce2Cu I2 - 2CuI May I know why the 2nd method direct synthesis is better than the 1st in terms of green chemistry. Di antara spesi berikut ini yang paling tidak mungkin digunakan sebagai. Многие реакции протекают только в растворах и не протекают между твердыми веществамиЧтобы в этом.
 Source: studylibid.com
Source: studylibid.com
Tentukan apakah reaksi 2CuSO4 4KI 2CuI I2 2K2SO4. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations. 1 1 iii In terms of electron transfer explain why copper is reduced in this reaction. با تشكر - تبیان.
 Source: youtube.com
Source: youtube.com
Cu2 changed to Cu means gains electron 1 iv Identify the reducing agent. Click hereto get an answer to your question In the reaction 2CuSO4 4KI Cu2I2 2K2SO4 I2 the equivalent weight of CuSO4 is. Di antara spesi berikut ini yang paling tidak mungkin digunakan sebagai. 2cuso 4 4ki 2cui i 2 2k 2 so 4 Check the balance Copper sulfate react with potassium iodide to produce copperI iodide iodine and potassium sulfate. 2 cu 2 s 6 o-2 4 4ki 2e- 2h 2 cu 1 i-1 2k 2 so 4 i 2 Balanced half-reactions are well tabulated in handbooks and on the web in a Tables of standard electrode potentials.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Click hereto get an answer to your question In the reaction 2CuSO4 4KI Cu2I2 2K2SO4 I2 the ratio of equivalent mass of CuSO4 to its molar mass is. 2CuSO44KI2CuII22K2SO4Zat yang berperan sebagai oksidator. The solubility product of Ag2C2O4 at. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol. 4KI 2CuSO4 —– 2CuI I2 2K2SO4.

2CuSO4 4KI 2CuI I2 2K2SO4 Cu 2 1 reduksi I -1 0 oksidasi Reduktor KI Oksidator CuSO4 Hasil reduksi CuI Hasil Oksidasi I2 b. 2CuSO4 4KI 2CuI I2 2K2SO4 Cu 2 1 reduksi I -1 0 oksidasi Reduktor KI Oksidator CuSO4 Hasil reduksi CuI Hasil Oksidasi I2 b. Unsur iodin dalam senyawa dapat ditemukan dengan biloks dari -1 sampai 7. Cu2 changed to Cu means gains electron 1 iv Identify the reducing agent. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations.
 Source: youtube.com
Source: youtube.com
Cu2 changed to Cu means gains electron 1 iv Identify the reducing agent. Tentukan apakah reaksi 2cuso4 4ki 2cui i2 2k2so4 merupakan reaksi redoks atau bukan. Di antara spesi berikut ini yang paling tidak mungkin digunakan sebagai. Cu2 changed to Cu means gains electron 1 iv Identify the reducing agent. با تشكر - تبیان.
 Source: brainly.co.id
Source: brainly.co.id
Mw 1595g mol-1. The solubility product of Ag2C2O4 at. 4KI 2CuSO4 —– 2CuI I2 2K2SO4. Enter an equation of a. 2CuSO4 4KI 2CuI I2 2K2SO4 Experts please explain how Cu2 changed to Cu1 in above reaction - Science - Chemical Reactions and Equations.

با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator. These tables by convention contain the half-cell potentials for reduction. با تشكر - تبیان.
 Source: breslyn.org
Source: breslyn.org
2cuso 4 4ki 2cui i 2 2k 2 so 4 Check the balance Copper sulfate react with potassium iodide to produce copperI iodide iodine and potassium sulfate. 2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator. Zat yang bertindak sebagai zat oksidator adalah. با تشكر - تبیان. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations.

2cuso4 4ki 2cui i2 2k2so4 The free iodine is titred against standard solution of sodium thiosulphate hypo using starch as an adsorption indicator. با تشكر - تبیان. Di antara spesi berikut ini yang paling tidak mungkin digunakan sebagai. Identify The Oxidizing And Reducing Agents In Each Equation Assign Oxidation Numbers To Each And Write Balanced Net Ionic Equations. Cu2 changed to Cu means gains electron 1 iv Identify the reducing agent.

2CuSO4 4KI 2CuI I2 2K2SO4 Experts please explain how Cu2 changed to Cu1 in above reaction - Science - Chemical Reactions and Equations. Enter an equation of a. These tables by convention contain the half-cell potentials for reduction. با سلام مسIIسولفات مطابق معادله زیر با پتاسیم یدید واكنش می دهد. The solubility product of Ag2C2O4 at.
 Source: youtube.com
Source: youtube.com
Click hereto get an answer to your question In the reaction 2CuSO4 4KI Cu2I2 2K2SO4 I2 the ratio of equivalent mass of CuSO4 to its molar mass is. Unsur iodin dalam senyawa dapat ditemukan dengan biloks dari -1 sampai 7. Click hereto get an answer to your question In the reaction 2CuSO4 4KI Cu2I2 2K2SO4 I2 the equivalent weight of CuSO4 is. 1 4CuOs CH4g 4Cus CO2g 2H2Ol 2 2CuSO4aq 4KIaq 2CuIaq 2K2SO4aq I2aq 3 Cu2Os Fe2SO43aq H2SO4aq 2CuSO4aq 2FeSO4aq H2Ol. با تشكر - تبیان.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 2cuso4 4ki 2cui i2 2k2so4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





